Elements and Compounds
An Atom
- Atom - smallest unit of all matter, that is composed of 3 sub-atomic particles called protons, electrons and neutrons
- Proton - the 'heavy' positively-charged particle in the nucleus of an atom
- Electron - the very 'light' negatively-charged particle that orbits the nucleus of an atom
- Neutron - the 'heavy' uncharged particle in the nucleus of an atom
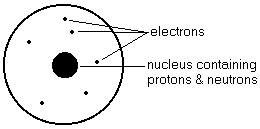
- Uncharged or unreacted atoms have the same number of positive protons and negative electrons.
- Approximate size of atoms - Millions of atoms could fit on the sharp point of a needle. Also, if you imagine that an atom is the size of an oval, a proton and a neutron would be the size of footballs in the middle of the oval, and the electron would be the size of a rice grain racing around the running lane.
Atomic Number and Atomic Mass
- An Example from the Periodic Table
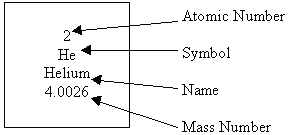
- Atomic Number - the number of protons in an unreacted atom
- Mass Number - the number of protons and neutrons together
Atomic Diagrams
- Protons and neutrons are in the nucleus.
- Protons are p+
- Neutrons are n
- Electrons orbit the nucleus in electron levels or 'rings'.
- Electrons are e–
- A limited number of electrons are situated in each electron 'ring'.
- First ring - maximum of 2 electrons
- Second ring - maximum of 8 electrons
- Third ring - maximum of 8 electrons
- Fourth ring - maximum of 18 electrons
- Electron rings close to the nucleus are filled first.
Elements and Compounds

- Element - An element is a substance composed of the same type of atoms (e.g. gold Au, oxygen O2).
- Compound - A compound is a substance made of more than one type of atom (e.g. water H2O, carbon dioxide CO2).
- Molecule - A molecule is the smallest particle of either an element or a compound.
Inert or Noble Gases
- Inert or Noble Gases are unreactive gases. They do not corrode or react.
- Examples of Noble Gases are:
- He - Helium
- Ne - Neon
- Ar - Argon
- Kr - Krypton
- Xe - Xenon
- Rn - Radon
- The electron rings of these unreactive gases are full, therefore they become stable.
Ions (Charged Atoms)
- When atoms react, they may either gain or lose electrons. Electrons have a negative charge. An atom gaining or losing electrons will get an overall charge.
- Positive Ions are atoms that have lost electrons (e.g. sodium Na1+)
- Negative Ions are atoms that have gained electrons (e.g. chlorine Cl1–)
- In chemical reactions, atoms tend to gain or lose electrons to resemble the electron numbers of the stable Noble Gases.
Covalent and Ionic Compounds
- Covalent Compound- a compound where electrons are shared between the atoms (e.g. carbon dioxide CO2)
- Ionic Compound - a compound formed from the attraction between positive and negative ions. For example in the ionic compound sodium chloride NaCl, the chlorine ion (Cl1–) gains one electron that was given by the sodium ion (Na1+).
Common Elements and Symbols to Learn
| Element Symbol | Element Name | Element Symbol | Element Name |
| H | Hydrogen | Mn | Manganese |
| He | Helium | Fe | Iron |
| Li | Lithium | Co | Cobalt |
| C | Carbon | Ni | Nickel |
| N | Nitrogen | Cu | Copper |
| O | Oxygen | Zn | Zinc |
| F | Fluorine | Br | Bromine |
| Ne | Neon | Ag | Silver |
| Na | Sodium | Sn | Tin |
| Mg | Magnesium | I | Iodine |
| Al | Aluminium | Ba | Barium |
| Si | Silicon | W | Tungsten |
| P | Phosphorus | Pt | Platinum |
| S | Sulphur | Au | Gold |
| Cl | Chlorine | Hg | Mercury |
| Ar | Argon | Pb | Lead |
| K | Potassium | Cr | Chromium |
| Ca | Calcium | Ti | Titanium |
| Pu | Plutonium | U | Uranium |
Naming Compounds
| PREFIX OR SUFFIX | MEANING | EXAMPLE |
| Mono- | There is 1 atom of that type in that molecule | Carbon monoxide (CO) |
| Di- | There are 2 atoms of that type in the molecule | Carbon dioxide (CO2) |
| Bi- | Hydrogen is present in the molecule | Sodium bicarbonate (NaHCO3) |
| -ide | There are only 2 types of atoms present in the molecule | Lead oxide (PbO) |
| -ate | There are 3 or more types of atoms in the molecule, and 1 type is oxygen | Calcium carbonate (CaCO3) |
Valency Table
- Valency - the charge of an ion or radical which has either lost or gained electrons
- Note that metals lose electrons easily to become positive ions. This is why most metals are good conductors of electricity.
| 1+ | 2+ | 3+ | 1– | 2– | 3– |
| H 1+ | Mg 2+ | Al 3+ | F 1– | O 2– oxide |
PO4 3– phosphate |
| Na 1+ | Ca 2+ | Fe 3+ ferric |
Cl 1– | S 2– sulphide |
|
| Li 1+ | Cu 2+ | Br 1– | CO3 2– carbonate |
||
| K 1+ | Zn 2+ | OH 1– hydroxide |
SO4 2– sulphate |
||
| Ag 1+ | Pb 2+ | NO3 1– nitrate |
|||
| NH4 1+ ammonium |
Fe 2+ ferrous |
HCO3 1– bicarbonate |
Working Out Formulae of Ionic Compounds
(The Cross-Over Method)
- Step 1 - In the ionic compounds to be learnt in junior science, there are two parts to the ionic compound - the first is a positive ion (usually a metal e.g. Na1+) and the second is a negative ion (e.g. Cl1–).
- Step 2 - Using the valency table, write the two ions and their valencies.
- Step 3 - Now ignore the positive and negative signs. Cross-over the top valency number to the bottom of the other ion symbol. Do this for both.
- Step 4 - Write the completed formulae with those same numbers at the bottom.
- Step 5 - If the numbers on each part are the same (e.g. Na1 Cl1 or Mg2 O2), ignore them and rewrite the formulae without them (e.g. Na Cl or Mg O).
- Step 6 - Brackets may be used around radicals (groups of atoms that are charged e.g. CO3).

Examples of Chemical Names of Compounds
| CHEMICAL FORMULA | CHEMICAL NAME |
| C O2 | carbon dioxide |
| C O | carbon monoxide |
| Na Cl | sodium chloride |
| Cu O | copper oxide |
| Ag Br | silver bromide |
| K I | potassium iodide |
| H Cl | hydrogen chloride (hydrochloric acid) |
| NH4 Cl | ammonium chloride |
| K OH | potassium hydroxide |
| Na OH | sodium hydroxide |
| Ca (OH)2 | calcium hydroxide |
| Ca S | calcium sulphide |
| Na NO3 | sodium nitrate |
| H NO3 | hydrogen nitrate (nitric acid) |
| Na HCO3 | sodium bicarbonate |
| Zn SO4 | zinc sulphate |
| Mg CO3 | magnesium carbonate |
| Ca SO4 | calcium sulphate |
| Cu CO3 | copper carbonate |
| Al PO4 | aluminium phosphate |
| Fe SO4 | iron sulphate |
| Fe CO3 | iron carbonate |
| NH4 NO3 | ammonium nitrate |
| NH4 HCO3 | ammonium bicarbonate |
| H2 SO4 | hydrogen sulphate (sulphuric acid) |
| Na2 SO4 | sodium sulphate |
| (NH4)2 CO3 | ammonium carbonate |
Examples of Numbers and Types of Atoms
| NAME OF SUBSTANCE | CHEMICAL FORMULA | ELEMENT OR COMPOUND | NUMBER AND TYPE OF ATOMS IN MOLECULE |
| Hydrogen | H2 | element | 2 hydrogen atoms |
| Carbon dioxide | CO2 | compound | 1 carbon atom 2 oxygen atoms |
| Water | H2O | compound | 2 hydrogen atoms 1 oxygen atom |
| Methane | CH4 | compound | 1 carbon atom 4 hydrogen atoms |
| Sodium hydroxide | NaOH | compound | 1 sodium atom 1 oxygen atom 1 hydrogen atom |
| Calcium hydroxide | Ca(OH)2 | compound | 1 calcium atom 2 oxygen atoms 2 hydrogen atoms |